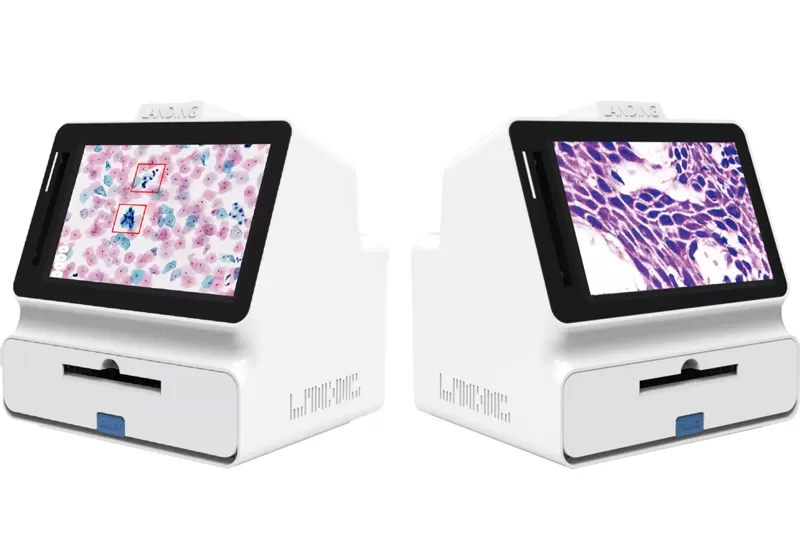
This whole slide imaging scanner seamlessly integrates with Landing Med's cervical cytology Al system.
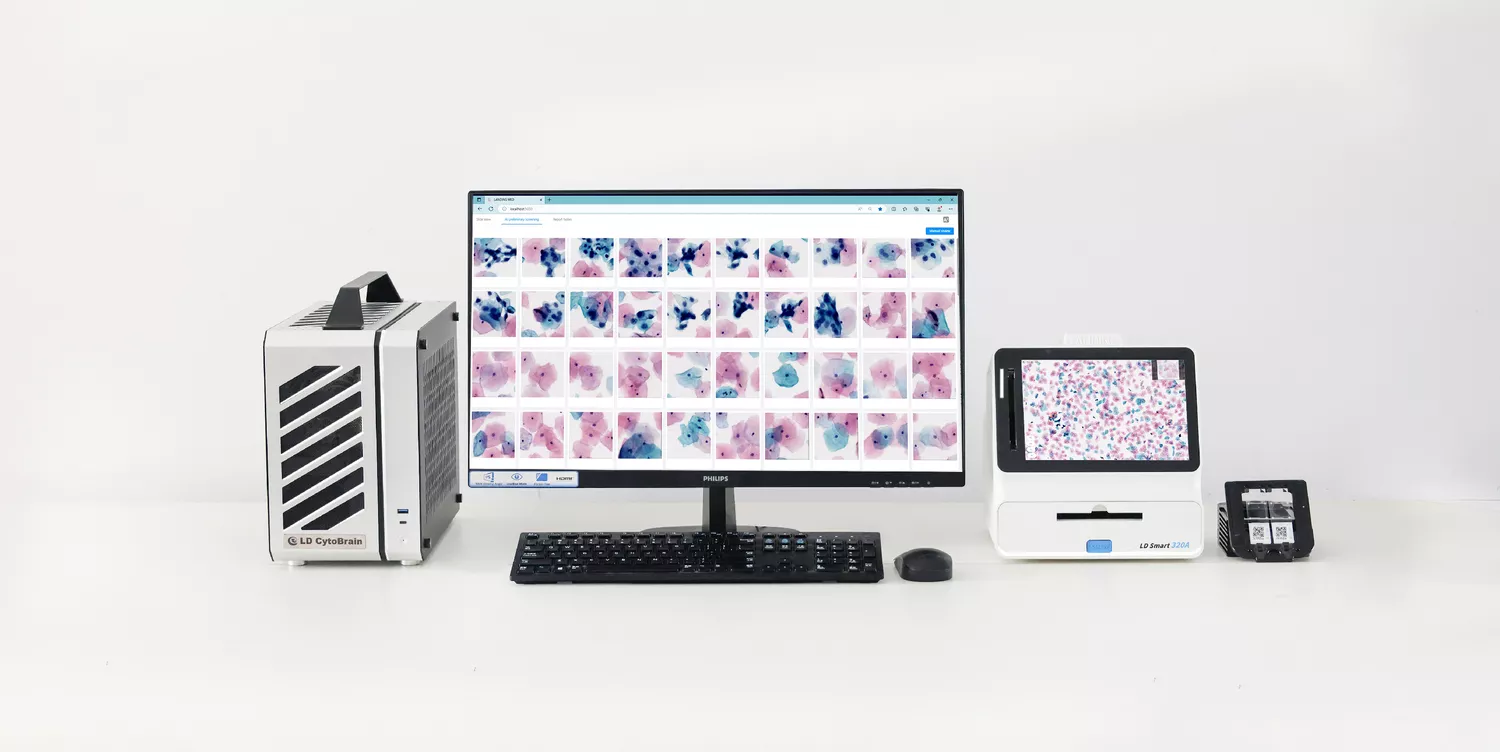
Mode: Brightfield scanning
Capacity: 2 slides
Magnification: 20X
Share: Share scanned slides via LAN
LD Patho 320A is CE-IVD certified in conformity with Regulation (EU) 2017/746 of the European Parliament and of the Council of 05 April 2017 on in vitrodiagnostic medical devices. Landing Med's solutions including AI-assisted cervical cytology system is for research use only, not for use in diagnostic procedures. Research use only in US.
